Metabolic and Weight Changes With Apremilast in Patients With Psoriasis. Pooled Laboratory analysis of the Phase 3 ESTEEM 1 and 2 Trials
Armstrong AW et al (Mulitcenter Study)
Poster 1652: EADV Annual Meeting 2015 – Copenhagen, Denmark
-Small molecules are the latest* in the treatment of psoriasis and psoriatic arthritis
Apremilast (Otezla):
-Is an inhibitor of phosphodiesterase 4 (PDE4)
-It has the advantage of being taken orally as 1 tablet of 10mg taken twice a day.
-Although few side effects are reported, weight loss has been observed in earlier studies and this is why this FDA-approved drug is contraindicated in patients with a Body Mass Index (BMI) under 20.
Results
–16 weeks after starting the treatment: mean weight loss was of 1.52 Kg, although 13.7% of patients had experienced weight loss of more than 5% of total body weight. (Total of 742 patients)
–52 weeks after starting the treatment, mean weight loss was 1.95 Kg, although 19.2% of patients had lost more than 5% of total body weight. (Total of 462 patients)
-For the sake of completeness: regarding sugar levels (glycated hemoglobin), there is a 0.4% decrease for patients on apremilast after 16 weeks of treatment – however there is also a decrease (0.2% for patients) on placebo.
Comments
-The results are based on the phase 3 studies which were made to validate the drug. it is therefore a retrospective study…and although not focussing on weight it did measure the effect specifically.
-The resulting weight, loss although statistically significant, is small although further prospective studies might show a bigger difference, as it is observed by many clinicians in practice.
*at the time of publication


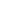

 +41 22 738 18 48
+41 22 738 18 48